

Stroke patients attended by mobile stroke imaging are 46% more likely to have no residual symptoms when assessed 90 days after treatment, compared to patients attended by regular emergency services [1]. However, the cost-effectiveness of current Mobile Stroke Units is closely tied to population density, exacerbating the healthcare equity gap between rural and urban patients [2][3].
Micro-X is helping to close this gap, bringing equity to remote patients, by developing a lightweight mobile stroke imaging solution that can be installed into regular road and air ambulances, without hindering their ability to attend non-stroke-related emergencies. This would help to decouple the cost effectiveness from population density, by allowing continued vehicle productivity during scanner downtime.

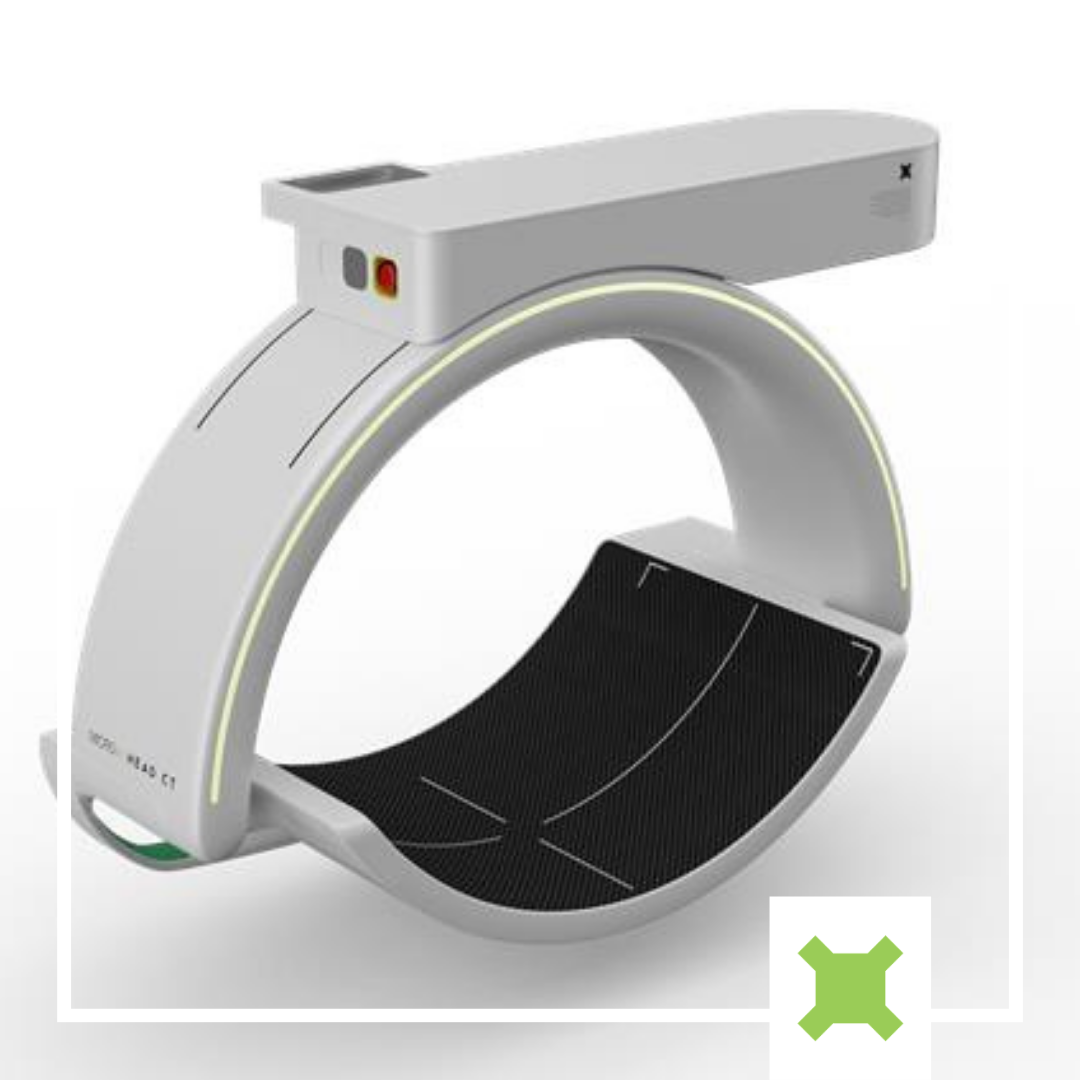
We developed our first head CT prototype in 2019 and completed cadaver testing at the Melbourne Brain Centre at the RMH. Since then, we have partnered with Johns Hopkins University to refine every aspect of the device, from physical configuration to reconstruction software, building five separate prototype devices along the way (see Figure 1). The latest prototype devices, which include genuine curved-panel detectors and incorporate discrete system motion, have produced high-quality images, capable of differentiating brain structures and detecting small structures in phantom bench studies (See Figure 2).

The performance improvements we have achieved since the first prototype in 2019 are the result of multiple key factors. From a hardware perspective, the addition of 6 mini-CNT x-ray sources in the multi-source array (for a total of 21) and the inclusion of discrete rotational indexing, contribute to greater sampling density and angular coverage of the head without significantly increasing the weight or footprint of the device. Systematic analysis and optimization of geometric parameters [4] achieved greater sampling uniformity, ensuring sufficient resolution of structures in all regions of the brain.
The imaging software pipeline we have developed, alongside Johns Hopkins University’s I-Star Labs, for artifact correction and image reconstruction addresses the imaging challenges associated with our novel configuration. Notably, Adaptive Deep Scatter Estimation (ADSE) surpasses gold-standard scatter correction performance, while achieving a ~1000x reduction in computation time [5], eliminating a major source of risk for implementation in time-critical scenarios.
Phantom imaging on our 2024 prototype is in progress. Once completed and subject to receiving ethics approval, we will proceed to live patient imaging trials.

[1] J. C. Grotta et al.,“Prospective, Multicenter, Controlled Trial of Mobile Stroke Units,” NewEngland Journal of Medicine, vol. 385, no. 11, pp. 971–981, Sep. 2021, doi:10.1056/NEJMoa2103879
[2] S. Walter et al., “Mobile Stroke Units - Cost-Effective or Just an Expensive Hype?,” Curr Atheroscler Rep, vol. 20, no. 10, p. 49, Oct. 2018, doi: 10.1007/s11883-018-0751-9.
[3] Z. Schofield, A. Balabanski, A.Place, P. Sharma, F. Gardiner, “The Stroke Report: Equitable care everywhere,”Apr. 2022, doi: 10.13140/RG.2.2.27490.81609.
[4] A. Lopez Montes, T. McSkimming,W. Zbijewski, J. H. Siewerdsen, C. Delnooz, A. Skeats, B. Gonzales, A. Sisniega, "Stationary x-ray tomography for hemorrhagic stroke imaging: sampling and resolution properties," Proc. SPIE 12304, 7thInternational Conference on Image Formation in X-Ray Computed Tomography, 123040P (17 October 2022); https://doi.org/10.1117/12.2646858
[5] T. McSkimming, A. Lopez-Montes,A. Skeats, C. Delnooz, B. Gonzales, E. Perilli, K. Reynolds, J. H. Siewerdsen,W. Zbijewski, A. Sisniega, "Multi-source semi-stationary CT for brain imaging: development and assessment of a prototype system and image formation algorithms," Proc. SPIE 12925, Medical Imaging 2024: Physics of Medical Imaging, 129251B (1 April 2024); https://doi.org/10.1117/12.3006970

Hear from CEO Kingsley Hall on the latest developments with the Head CT Scanner for stroke


Australia’s manufacturing future will be defined by modular systems, biotech integration and smart partnerships that empower SMEs to innovate, adapt and thrive.


From CT scans in ambulances to virtual hospitals, new Aussie tech and innovations are improving tests, treatment, and are set to bring higher quality care to Australia.

Micro-X creates revolutionary X-ray technology to better lives.
Our Purpose
Find out how Micro-X is creating new opportunities for industries across the world.
Find out more
They’re the visionaries and innovators behind our X-ray technology, products, culture and ethos.
Meet the team