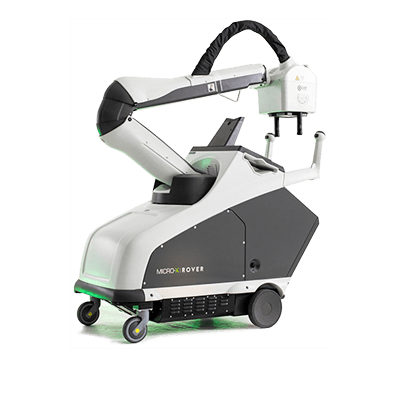
Micro-X Ltd is an award winning ASX listed X-ray technology company. We are pioneers of a global revolution in medical and security imaging products using cold cathode X-ray sources.
Micro-X is on a mission to build an inclusive future that benefits our employees, communities and the planet. We’re not focusing on how we can change the world overnight, but what we can do today that will take us one step closer to our goal.