Rover Mobile X-ray FDA clearance
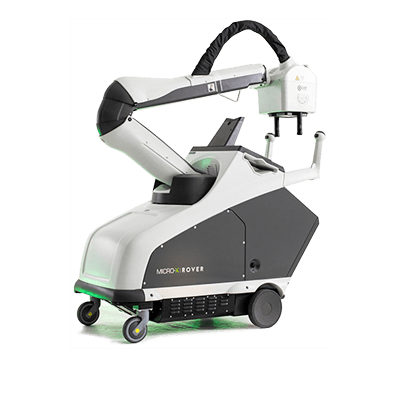
Rover Mobile X-ray US FDA 510(K) Clearance Micro-X is pleased to announce it has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its ‘Rover’ mobile X-ray product which is designed for deployed military medical facilities. Click...
Rover Mobile X-ray US FDA 510(K) Submission
Rover Mobile X-ray US FDA 510(K) Submission Micro-X’s Managing Director, Peter Rowland, commented: “We are very pleased to have submitted Micro-X’s first 510(k) application under its own name to the FDA. Obtaining FDA regulatory clearance is a key milestone of...
NWR Virtual Cap Health Conference recording
Micro-X is pleased to advise that you can register to watch Peter Rowland, Managing Director’s presentation at the NWR Virtual Small Cap Health Conference held on Friday 1 May 2020. REGISTER to watch the recording on-demand